
The pharmaceutical industry in the United States is red hot, with consumers spending more money on prescription drugs, over-the-counter meds, and other healthcare products in the States than anywhere else.
Given the demand, it sounds like a good industry to be in, doesn’t it?
However, there’s one distinct drawback, and that’s the ever-changing pharma advertising regulations.
The problem is, it’s so easy to overstep the mark with your pharma advertising. Once you stray into that territory, that could be your credibility shot. And it may lead to a whole heap of problems from the FDA.
That’s the reason for this post.
I’ll try to keep you on the straight and narrow so you successfully adapt to changes and continue to thrive.
Ready? Here we go.
Key Points
- Digital marketing for the pharmaceutical industry is heavily regulated, with strict rules for every promotional content governing what can be said, how it is said, and to whom it can be said.
- Pharmaceutical companies must comply with guidelines set by platforms like Google Ads and regulations specific to their country or state.
- New regulations roll out on May 20, 2024, with a compliance date of November 20, 2024.
- Marketers should provide accurate info about benefits and risks, use clear and understandable language, and avoid promoting medicines for off-label uses.
- Keep communication open, address any concerns or questions the FDA may have from FDA reviews, conduct risk management, and offer continuous training to staff.
Understanding Evolving Regulations
The FDA began regulating prescription drug advertising and labeling in 1962. Back then, the main outlets for marketing were print and broadcast media.
Today, pharma advertising involves many more marketing channels like apps, video, social media, mobile, and traditional print and broadcast. According to Statista, the U.S. is the market leader in pharma marketing with a 42.6 percent market share, accounting for almost half of the worldwide revenue.
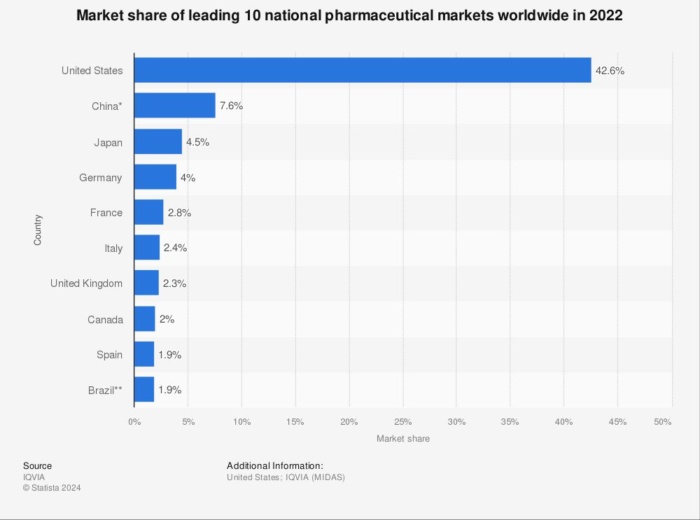
The FDA regulates promotional content, product labeling, packet inserts, and clinical trial data. Its regulations state that ads must highlight a pharmaceutical product’s risks and benefits, and it will act if it considers a company is making false or misleading claims.
Away from traditional advertising, times have changed, and the FDA has had to keep pace with Direct-to-Consumer Advertising (DTCA). Initially, the agency hesitated to allow TV advertising, fearing it would lead to misleading promotions. However, the FDA has recognized the benefits of DTC advertising and its role in public health.
One area of focus for the FDA is digital advertising. As this chart from Insider Intelligence shows, U.S. healthcare and pharma digital ad spending is already at record highs.
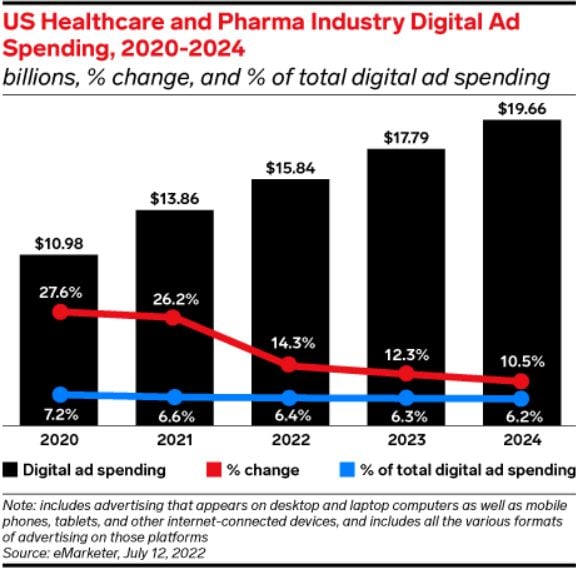
In 2024, digital marketing will continue playing a significant role in the pharmaceutical industry, as around 90 percent of pharma leaders prioritize digital channels.
To keep pace with the digital world, the FDA has comprehensive guidelines for making effective disclosures online and in PPC ads.
Summary of FDA Guidelines for Pharma Marketing
The FDA regulations cover various aspects of prescription drug advertising, including:
- Advertisement content
- Requirements for “brief summary,” “prescribing information,” “major statement,” and “adequate provision.”
- Ad design guidelines for marketing promotions related to:
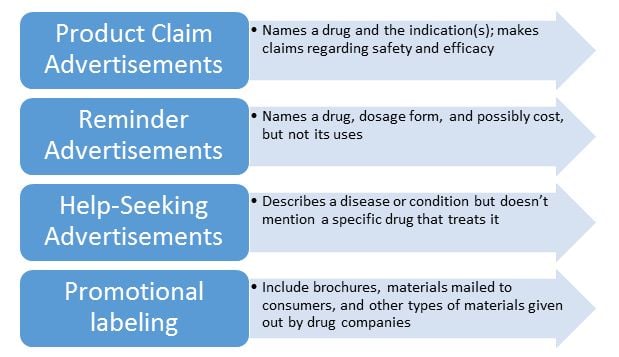
The FDA’s advertising guidelines apply to all qualifying pharmaceutical products and medical device classifications. Here’s a summary:
- Regularly monitor and stay updated on FDA guidelines related to pharma advertising.
- Ensure your promotional materials align with FDA-approved product labeling.
- Use clear, understandable language in promotional materials.
- Maintain a balanced presentation of your product’s benefits and risks in promotional materials.
- Avoid promoting medicines for off-label uses.
- Follow specific FDA and PhRMA guidelines regarding DTC pharma advertising.
- Monitor and report adverse events related to the promoted product.
- Uphold ethical standards in all promotional activities.
Finally, continuously improve by assessing and enhancing internal processes, education, and procedures based on evolving FDA guidelines and industry best practices. You should always look for ways to improve compliance and build brand trust and credibility.
Updates in Pharmaceutical Advertising Guidelines
Recently, the FDA announced new regulations for DTC advertising, and pharma companies must now include the “Major Statement” in their advertising. It must also:
- Be in easy-to-understand language
- Ensure related audio content is understandable
- Avoid using audio or graphic elements that might affect consumers’ understanding of an advert
- Provide easy-to-read text in any television advertising
- Show audio and text at the same time in TV ads and give consumers enough time to understand it
These rules come in on May 20, 2024, with the compliance date on November 20, 2024.
Navigating FDA Guidelines
Successfully navigating changing FDA guidelines is vital for individuals and companies developing, approving, and advertising medical products.
Although the guidelines may seem like a lot to take in, you can break it down into stages, like this:
- Choose the correct FDA classification: The FDA has three categories. Class I covers items like basic wheelchairs, bandages, and other low-risk products. Class II is for moderate-risk items, and Class III is for high-risk items.
If you’re unsure about classification, follow the FDA guidance to check guidelines and see if the FDA classes your product as a medical device.
- Verify product safety: Conduct a comprehensive research program, including preclinical guidelines, clinical trials, and data collection. The FDA sets out guidelines for successful study design and execution.
- Ensure Good Manufacturing Practices (GMP): These standards enhance the quality and consistency of medical products. A robust GMP system limits risks during manufacturing and maintains quality standards.
- Submit regulatory documents: This includes forms like the Investigational New Drug Application, Premarket Approval for new medical devices, and New Drug Applications. Also, refer to FDA guidance to check if you need to file any further documents.
- Keep communication open: Address any concerns or questions the FDA may have from FDA reviews.
Impact Pharma Advertising Regulations Has on Marketing Strategies
Perhaps the trickiest part of pharma advertising is getting the balance right. Of course, you want to shout about the benefits of your product. But you also want to reign in any claims you can’t support and keep on the right side of regulations.
FDA regulations prioritize patient safety, scientific accuracy, and transparency. This means you’ll need to think carefully about your language and images while spreading the word about your product’s advantages. Remember, compliance with FDA guidelines is not only a legal requirement. It’s crucial for building and maintaining trust in your brand.
It can be a bit of a minefield understanding FDA regulations and how they shape your marketing strategies, so I’ve included some key considerations below:
Labeling and Advertising Restrictions
FDA regulations impose strict drug labeling and advertising guidelines. You’ll need to provide accurate information about your product’s benefits and risks, ensuring promotional materials are truthful and not misleading. For example, you should support promotional messages with scientific evidence that does not overstate the benefits or downplay the risks of a drug.
This ad for Orserdu on social media uses an on-demand broadcast demonstrating the safety and efficacy of the treatment by providing data-backed evidence.

DTC Pharma Advertising
While the FDA permits direct-to-consumer advertising, it has specific requirements. Advertisements must include benefits and risks and should be clear and not misleading. Carefully craft your promotional materials to comply with FDA regulations while effectively reaching and educating consumers about your products.
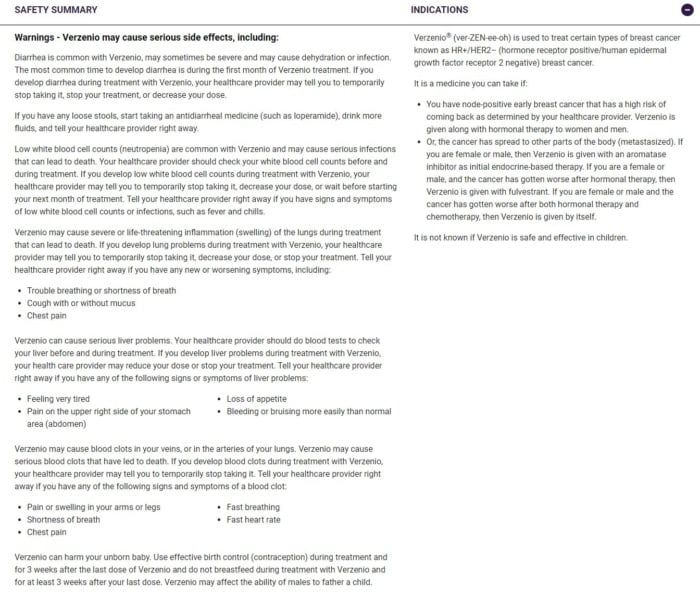
This advert for Verzenio gets the balance right by detailing the benefits of the drug but also highlighting the potential side effects.
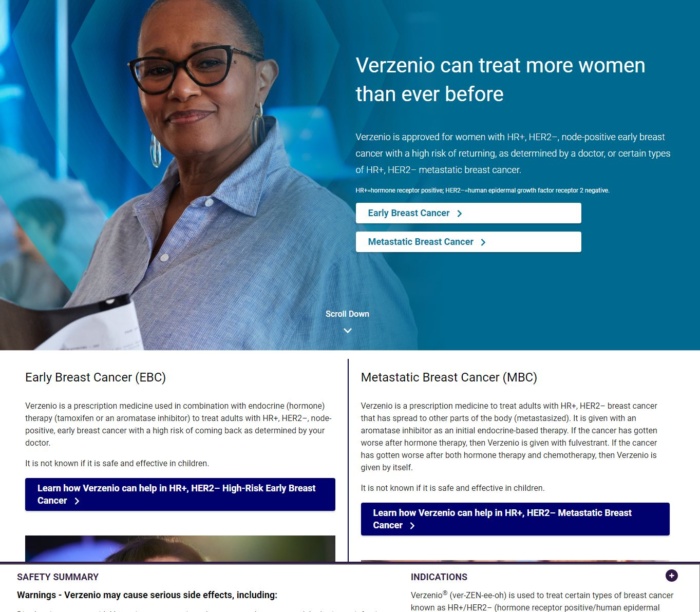
It uses plain language and avoids overwhelming readers by adding an expandable safety summary at the bottom of the webpage:
Social Media and Digital Marketing
Social media and digital marketing have presented new opportunities and challenges for pharmaceutical companies. However, the FDA has guidance about using these platforms while staying within regulatory boundaries.
This advertisement from Bayer shows how gene therapy could help manage the symptoms of Parkinson’s disease. Note the careful choice of language, using terms like “could” rather than “will.” Keeping the ad FDA compliant.
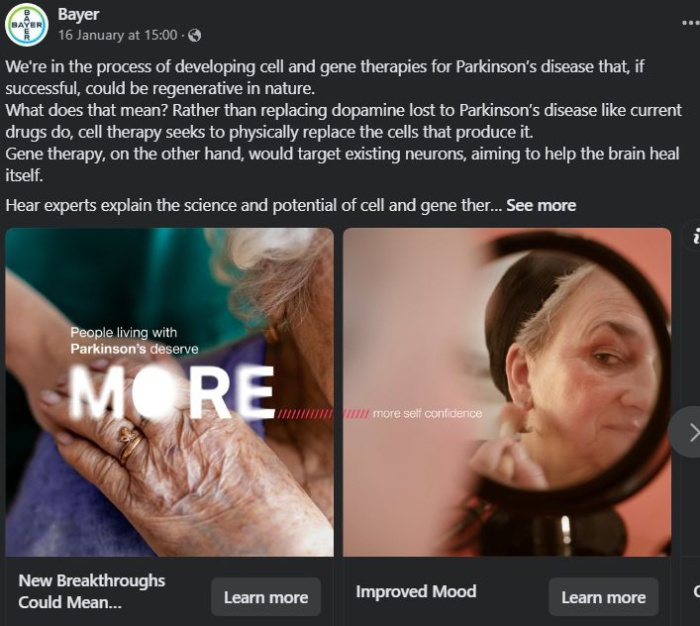
This ad avoids sharing too much information and allows consumers to click to find out more.
While social media and digital marketing channels enable you to reach a broader audience, you must still ensure regulatory compliance.
Additional Considerations for Marketers
In addition to various regulations I’ve already covered, the uncertainty and length of the FDA approval process can impact your marketing strategy. You’ll need to plan your promotional activities carefully, allowing for potential product launch delays.
Your marketing strategy should also involve educational initiatives for healthcare professionals, emphasizing the evidence-based benefits of drugs while adhering to strict guidelines regarding off-label use.
Finally, your marketing strategy must include plans for monitoring and responding to any emerging safety concerns. Additionally, adjust your promotional efforts in response to new information or regulatory guidelines.
Challenges Faced by Pharma Marketers
Strict regulatory requirements mean pharma marketing has always been challenging. But more recently, pharmaceutical advertising has come under increasing regulatory scrutiny from the FDA.
Medical brands are also contending with changing consumer expectations. Today’s consumers are more proactive and want to do what they can to manage their healthcare decisions and medications. If pharma brands are to reach these consumers, they must create personalized messaging while sticking to DTC pharma advertising guidelines.
Adapting to constant change is one of the biggest pitfalls of pharma advertising, but being aware of likely issues enables you to plan ahead and find solutions.
Some common challenges businesses face when adapting to regulatory changes include:
- Regulatory compliance: The FDA has strict regulations regarding marketing, and ads must adhere to accuracy and balance and disclose any risks. Regulatory compliance is made even more difficult by FDA updates that marketers need to keep pace with.
- Changing product compliance: New developments like cell therapy are exciting for the medical sector. While these products have huge potential, pharma companies must ensure their advertising communicates the risks and benefits.
- Reaching your target market: Whether you’re trying to reach patients or healthcare professionals, you must ensure you’re targeting and personalizing your message for each audience. One way to do this is through personalized email marketing that educates your customers and builds confidence.
- Establishing trust: It’s easy for consumers to be skeptical about product claims, so building a relationship with consumers is vital in pharma marketing. Brands must also strive to be transparent and keep consumers updated and informed about their products.
Best Practices for Pharma Advertising
As the way we absorb information evolves, regulatory bodies will likely introduce stricter guidelines for social media and working with influencers.
For instance, there are calls for the FDA and FTC to crack down on TikTok, Instagram marketers, and other influencers marketing prescription drugs with stricter regulations.
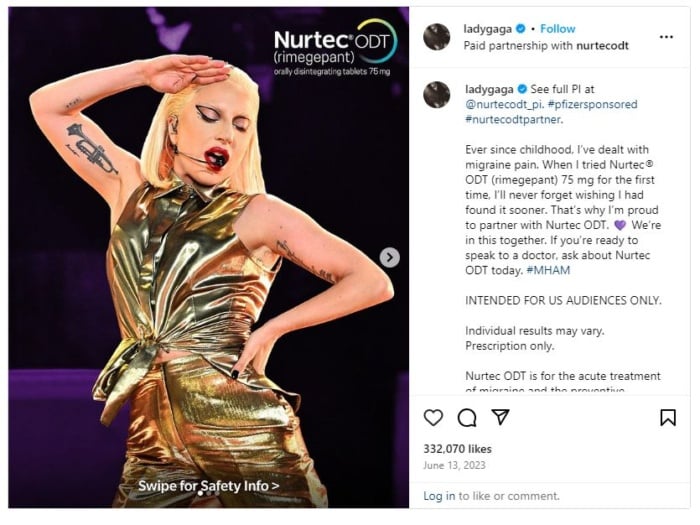
The key takeaway is that whichever advertising platform you use, double-check that it complies with the FDA.
Other best practices include:
- Stay up-to-date with regulations: with ever-changing guidelines, ensure you’re checking the FDA website, social media pages, and press release pages often. Conduct regular training sessions for marketing teams to ensure they are well-versed in FDA regulations.
- Help patients make informed decisions: Detail your medications’ benefits and possible risks in layperson’s terms and check marketing materials follow the FDA language guidelines.
- Substantiate product claims with evidence: Support any claims made in promotional materials with credible and scientific evidence.
- Work with regulatory bodies: Avoid common compliance mistakes by partnering with FDA regulatory agencies. The ProPharmaGroup.com is one example.
- Utilize review and approval processes: Implement robust review and approval processes for promotional materials involving legal, regulatory, and medical experts to catch potential compliance issues before distribution.
- Think security first: Use a secure platform to store your advertising content, communications with the FDA, and audit results.
- Use technology to manage your marketing: For example, Inzio Evoke is a system that assists with marketing and communication.
- Maintain comprehensive recordkeeping: Keep records of all promotional materials, communications, and related activities. This documentation serves as evidence of compliance in case of regulatory scrutiny.
FAQs
In the United States, pharma advertising regulations are constantly changing, with regular announcements that marketers need to stay on top of. In addition to new rules, there are amendments to previous regulations that marketers must keep in step with.
As detailed above, one of the major challenges is the frequent changes to pharma advertising regulations and what’s acceptable.
Ensuring regulatory compliance, winning customer trust, and adapting to a changing product landscape, like gene therapies, are considerable obstacles, too.
Along with pharma advertising regulations, the legal landscape of digital marketing is also evolving, with the onset of new AI-powered technologies and tools, changes to consumer privacy regulations, and paid media trends of 2023.
Some proactive strategies include having a compliance team and drawing up internal policies or guidelines to ensure your pharma advertising is compliant.
Keeping up with changing regulations by signing up for FDA press releases, training staff, and performing regular audits are also essential.
Direct-to-consumer (DTC) pharma advertising is when pharma companies market medications directly to the public. With pharmaceutical advertising in healthcare, the target market is healthcare professionals. In DTC pharma, companies reach out to consumers via online ads, print, commercials, and radio advertisements.
DTC pharma advertising aims to empower patients by informing them of available treatments and encouraging them to seek medical advice about their condition.
DTC advertising has become increasingly popular, and the FDA recently issued new guidelines for these types of consumer ads.
Conclusion
Every type of paid media comes with some risk, but pharma advertising is a legal landmine. Fortunately, you can take steps to keep on top of regulations.
One of the best places to start is the FDA website itself. It has a wealth of resources to get you on track, but pharma advertising is not an area where you can stand still.
FDA regulations are forever changing or adapting, especially with the growth of DTC marketing via social media channels. You must keep pace with them by regularly reviewing FDA guidelines, checking press releases, and working with a compliance team.
What do you find are the biggest challenges in pharma advertising?

